 I made a summertime trek to the Canadian Rockies to research an upcoming book and had some unexpected difficulties in the bathroom! My daily shower routine was disrupted because of hard water. I found that it was simply impossible to wash my hair with the stuff that was coming out of the shower nozzle.  Of course I had heard of hard and soft water before and have encountered places where rinsing off soap residue is a bit more challenging. But this was the first time that it was literally impossible to work my fingers into my wet hair to wash it.
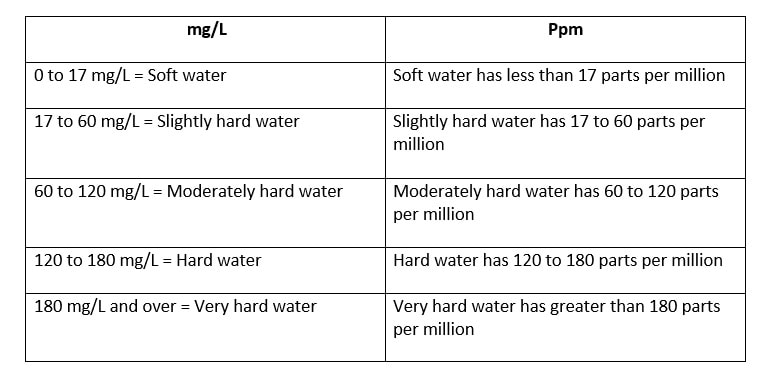
 The folks that I talked to had that spent their whole lives in Alberta and British Columbia and did not even realize that hair washing need not be such an angst-producing activity. I suspected the issue had to be hard water. I saw that I was correct once I figured out the best way to wash my hair while traveling in the Canadian Rockies. The Difference between Hard and Soft Water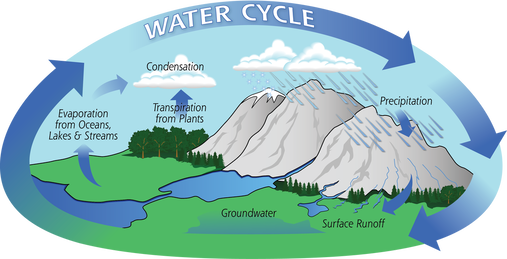 Rainwater falling from the sky is naturally soft, due to evaporation, condensation, and precipitation of the water cycle. But when it falls to the ground, it accumulates dissolved solids as it moves. When water picks up enough of certain minerals, it will become hard.  Moving through limestone, gypsum, chalk, and other rocks, hardens water. Igneous, sedimentary, and sandstones leave water soft. Depending on the geographical area, water may contain calcium, magnesium, sodium, and other minerals.  The hardness or softness of water is determined by the mineral content of both calcium and magnesium. Hard water contains an appreciable amount of calcium, magnesium, iron, and manganese ions usually in the form of bicarbonates, chlorides, and sulfates. Soft water has a minute amount of calcium and magnesium ions but contains sodium ions in an appreciable amount. In order to categorize water as soft or hard, the amount of calcium carbonate (CaCO3) in milligrams per liter (mg/L) or parts per million (ppm) is measured. Water begins to be considered hard if it has 17 or more mg/L (17 ppm) of calcium carbonate. So What?  Although hard water does not have any health concerns, it can be a nuisance as it interferes with almost every cleaning task, from doing the laundry to washing dishes and taking a shower. Hard water makes clothes feel rough and scratchy, shortening their lives. Spots may appear on dishes after drying. Hair becomes dull. Hard water can leave a residue buildup in pipes made of galvanized steel (characteristic of homes built from the mid-1940s through the 1970s), causing clogs. Hard water is more difficult to boil, increasing costs. Boilers using hard water become less efficient over time.  Soap lathers more easily with soft water. In hard water, soap forms insoluble curds, which result in spotty dishes, drying skin and hair, and deposition in pipes. A Matter of Preference?  Water quality varies in both hard and soft forms. Both types of water offer unique risks and benefits. Hard water contains more minerals, which are valuable nutrients for your body in the correct amounts. Minerals improve water’s taste.  Many people notice the mineral residue left by hard water. But soft water makes skin feel slippery because of the lack of minerals. That can feel like a residue, also.  Hard water does not pose any health risk and is, therefore, not regulated. The minerals in hard water are not contaminants, like germs or bacteria. The calcium and magnesium found in hard water can ensure meeting your daily requirement for these minerals.  The World Health Organization’s (WHO) Guidelines for Drinking Water Quality concluded that there was no firm evidence that drinking hard water causes any adverse effects on human health. The WHO does not give any requirements on municipal water softening or on the maintenance of residual calcium or magnesium levels.  Soft water contains a higher sodium content than hard water. Those following a low sodium diet due to high blood pressure would be wise not to drink too much of it. The solution, therefore, is to find a balance in the mineral content. Sabrina- Please fix this picture- there is something wrong with it. Test Your Water  Water hardness can easily be checked using a simple test. Not sure if you have hard water? You can get a general idea with this DIY: Fill an empty bottle half-way with some of your water. Next, add a few drops of liquid soap and shake the bottle. The fewer bubbles formed, the harder your water is. 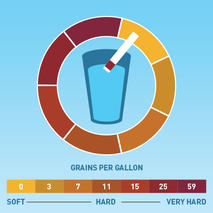 Use a hard water test kit to quantify the hardness level of water. A test kit works like a pH paper. An indicator paper is dipped in a water sample and the paper’s color change is matched against a chart. This test gives you the hardness of water in grains per gallon (GPG). Managing Hard Water  If you have hard water due to the presence of dissolved bicarbonate minerals (magnesium and calcium carbonates) in high enough levels, you may notice some negative effects. High levels of these minerals may have laxative effects and can cause diarrhea and renal issues. These dissolved bicarbonate minerals can be removed by boiling the water or by adding lime.  Removing spots on dishes and utensils can be achieved by simply drying with a soft cloth after washing with hard water. That will prevent mineral deposition.  Untreated calcium and magnesium that pour out of the showerhead will react with shampoos, conditioners, and soaps to reduce effectiveness and cause irritation. The more often you shampoo your hair in hard water, the less moisture that will be retained in the hair strands. This results in dry, coarse, frizzy hair, as well as a dry scalp and dandruff.  The minerals in hard water will mix up with the shampoo to form a scum which will settle on your scalp and hair follicles. This limescale will then prevent your hair from absorbing any moisture from the conditioner. Dry, straw-like hair causes unmanageable tangles, which makes hair susceptible to breakage and can lead to thinning. Hard water can also cause dyed hair to change colors, especially if there are high levels of other minerals like iron in the water.
Water Softener  Hard water can be managed. But, if you want long-lasting results, install a water softener. Water softeners work to remove calcium and magnesium in your home’s water supply. Soft water is produced by passing the hard water over an ion exchange resin, which consists of sodium salts. When hard water passes over the resin, positively charged salt ions replace the magnesium and calcium ions in the hard water. That is the reason why soft water has a high sodium ion content and tastes salty. Another benefit of using a water softener is that hard water caused by sulfates and chlorides of magnesium and calcium (which do not precipitate when heated and therefore, cannot be removed simply by boiling), can be removed with this technology.  Water softeners require regular maintenance to monitor clogs and clean the filtering mechanism. This is also important since bacteria and fungi may accumulate in the filter over time and need removal. Water softeners are not water filters. They don’t remove contaminants from water. Around 85% of the US water supply is hard, but many businesses and homeowners utilize water softeners to strip away extra minerals. Soft vs Deionized vs Distilled WaterSoft water contains lower levels of calcium and/or magnesium than hard water. It has less than 17 parts per million.  Deionized water is produced by passing the source water through a cation or anion exchange system, which removes all ions. Deionized water contains no minerals and is used for industrial and home cleaning where all impurities much be removed.  Distilled water is steam from boiling water that's been cooled and returned to its liquid state. It does not contain minerals. My Canadian Rockies Hard Water DIY Fix Since I was merely a visitor in BC and Alberta staying in inns and hotels I did not have the option of installing shower head filters or water softeners. I simply washed my hair with bottled water.  I was careful to keep my head out of the shower spray, which can be a challenge. And, pouring room temperature water over your head is pretty chilly. But it got the job done. I was able to massage in shampoo and conditioner, rinse, and run a comb through…none of which was possible with the hard, Rocky Mountain water!
0 Comments
Leave a Reply. |
AuthorGertrude Katz has spent over 30 years teaching K-12 public school students all major subjects. She has taught biology and education at the college level. The majority of her career has been spent instructing biology at the secondary level. Categories
All
|




 RSS Feed
RSS Feed