
Try to replicate the activity pictured here in your classroom! Get these supplies:
Observe & predict
How are the cans of soda alike? How are the cans of soda different? Which cans will float? Which cans will sink?
CHECK OUT MY TIKTOK VIDEO DEMONSTRATION BELOW, AND THEN READ ON FOR MORE DETAILS!
Procedure
Results
Students should observe that regular sodas with sugar sink and diet sodas float. Density
All the cans have the exact same shape, size, and volume, but they have different densities. The density of water is 1.0 grams per milliliter. Any object that has a density greater than 1.0 g/mL will sink and any object with a density of less than 1.0 g/mL will float.
You can determine how Dense an object is by using the equation: Density = Mass divided by Volume. There are two variables when figuring Density: Mass and Volume. Volume refers to how much space an object occupies. In fluids, volume is usually measured in liters (L) or milliliters (mL). All the cans used in this activity had the same amount of volume. The second variable in figuring Density is Mass. Determine the Mass of the Cans of Soda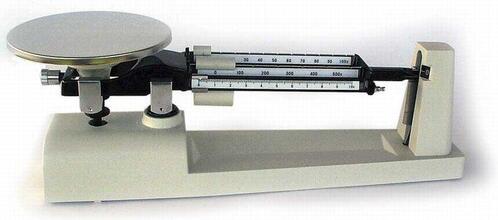
A scientific balance can determine the masses of both a can of regular soda and a can of diet soda. Mass refers to how much matter exists within an object; it is measured in grams. Students should find that regular soda has more mass than diet soda. The Reason for the Greater Mass of Regular Soda
Compare the list of ingredients on both the diet and regular cans of soda. You will note that the can of regular soda has over 150 calories while the diet soda has 0 calories. The calories in the regular soda come from sugar!
Diet sodas usually contain aspartame, an artificial sweetener, while regular sodas use sugar. Look again at the nutritional information on the cans. Most regular sodas have between 39 and 43 grams of sugar. This added mass is why the cans of regular soda sink in water. A sugar packet contains about 4 grams of sugar and about 15 calories. If a can of soda has 40 grams of sugar and 150 calories, it also contains 10 sugar packets. That is a lot of sugar, and many cans of soda contain more than that. The density of a can of soda depends on how much sugar or sweetener is used. The 40 grams of sugar in a can of regular soda makes it sink. The relatively small amount of artificial sweetener used in diet soda does not add much to its mass, enabling the can to float. Repeat the Experiment with Salt Water
Salt water is more dense than fresh water. Repeat this activity several times to see the amount of salt necessary to get both regular and diet soda to float. Here is an organized write-up of this activity to make it easy for your students to follow along!
0 Comments
Leave a Reply. |
AuthorGertrude Katz has spent over 30 years teaching K-12 public school students all major subjects. She has taught biology and education at the college level. The majority of her career has been spent instructing biology at the secondary level. Categories
All
|
 RSS Feed
RSS Feed