
You would think that if your cells made a poisonous chemical they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. Enzymes are not altered by the reactions. You have hundreds of different enzymes in each of your cells. Each of these enzymes is responsible for one particular reaction that occurs in the cell. 
Catalase is an enzyme that is found in the cells of many living tissues. It speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen.
This reaction is important to cells because hydrogen peroxide (H2O2) is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. 
You can teach your students about catalase by using chicken or beef liver from the grocery store. It might seem strange to use dead cells to study the function of enzymes. But when cells die, the enzymes remain intact and active for several weeks as long as the tissue is kept refrigerated

In the lab found in 40 Biology Lab Activities, students can investigate how the enzyme catalase reacts under environmental conditions such as variable pH and temperature. 
Surface area of both the enzyme and substrate also effect rate of the reaction. Greater surface area allows the substrate to react with a larger amount of the active site of the enzyme.
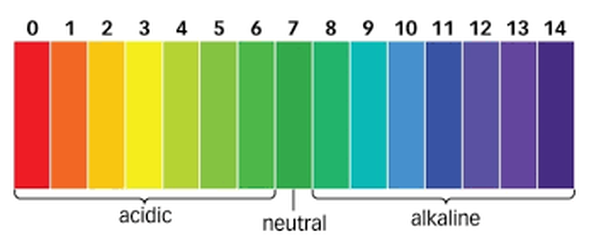
Under certain conditions enzymes can be denatured. An enzyme is denatured when the protein molecule loses its proper shape and cannot function. Some things that can denature an enzyme are enzyme concentration, extremes in temperature, and extremes in pH.
Sometimes students are squeamish about performing this lab because raw liver is yucky!!!
CHECK OUT MY TIKTOK VIDEO DEMONSTRATION BELOW, AND THEN READ ON FOR MORE DETAILS!
PROCEDURE:
Students use forceps, skewers, or chopsticks to position a piece of liver in a test tube. Then H2O2 is added to the liver, causing a chemical reaction. Bubbles of O2 are given off during an exothermic reaction. A glowing split can be relit from the gas that is released, demonstrating that is, in fact, O2.
Is Catalase Reusable?
Students discover that adding additional H2O2 produces new bubbles of O2, demonstrating that the catalase enzyme is reusable.
temperature, ph, and surface area
Students experiment with frozen, cooked, acidic, basic, and pulverized liver to determine what effect temperature, pH, and surface area have on catalase action. They find that the catalase in liver that has been exposed to temperature and pH extremes does not produce O2, but the pulverized liver with more surface area produces lots of O2.
0 Comments
Leave a Reply. |
AuthorGertrude Katz has spent over 30 years teaching K-12 public school students all major subjects. She has taught biology and education at the college level. The majority of her career has been spent instructing biology at the secondary level. Categories
All
|
 RSS Feed
RSS Feed