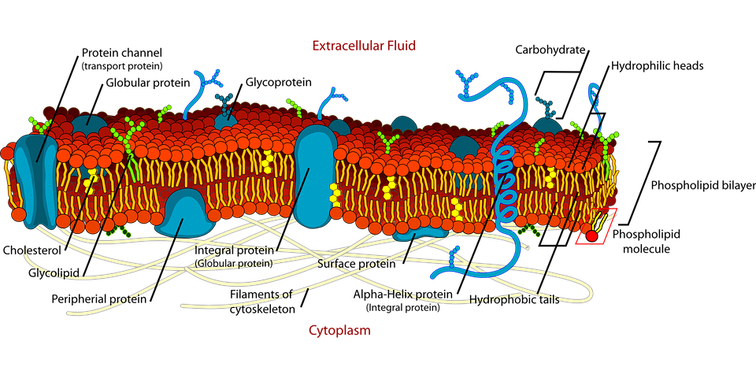
 Early farmers who discovered cheese in 8000 BCE used it as a dairy preservative, since cheese does not spoil as quickly as milk. But, by the 20th century, global trade necessitated an even longer shelf life. This spurred the invention of ultra-processed cheeses like Velveeta. To understand the science behind this udder marvel of chemistry and how you can use it in your classroom, it’s helpful to first learn about the components of milk. Milk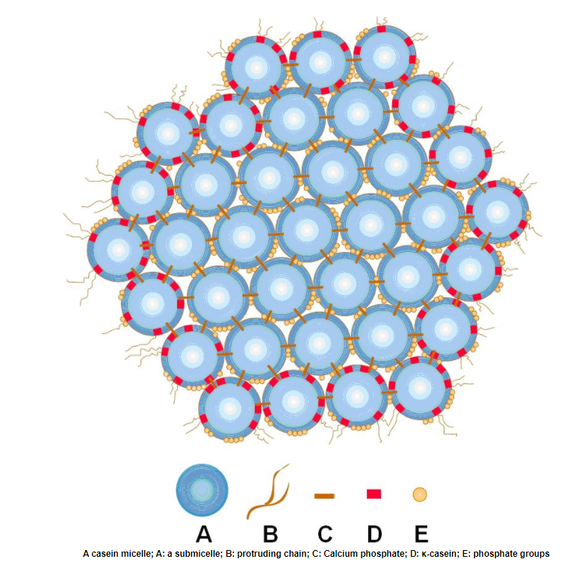 Depending upon the breed of cow and the season, milk is approximately 86% water, 4.5% fat, 5% carbohydrate (mainly lactose), 3.5% proteins (casein and whey), and less than 1% minerals and vitamins. The casein proteins are suspended in milk in structures called micelles. The outer part of the micelle, the Kappa casein, is hydrophilic, and it repels other casein micelles. Making Cheese is a Process  In cheese making, acidifying milk helps to separate the curds from the whey and control the growth of undesirable bacteria in cheese. Special ‘starter’ bacteria are added to milk to begin the cheesemaking process. These bacteria convert the lactose into lactic acid and lower the milk’s pH. Some cheeses are curdled only by acidity; Rennet is added to many other cheeses after the starter bacteria. Rennet speeds up the coagulation of casein and produces a stronger curd. It also allows curdling at a lower acidity, which is important for some types of cheese.  The chymosin enzyme in rennet binds to the Kappa casein and the micelles become hydrophobic. They attract and clump with other micelles. This forms a gel, which traps liquid whey and fat globules. When this gel is cut or stirred, cheese curds are formed. Continued cutting, salting, and draining off of whey forms different kinds of cheese. Biochemistry & The Study of Life Chemistry teachers can use milk as an example in talking about emulsions, suspensions, solutions, colloids, and mixtures. Homogenized milk is an oil-in-water emulsion, with the fat globules dispersed in a continuous phase of skim milk. If raw milk were left to stand, the fat globules would form a cream layer and rise to the surface. Homogenization of milk simply reduces the size of the fat globules mechanically to create a stable emulsion that has an increased shelf life.  Milk and cheese also make an interesting study in Biochemistry units within General Science and Biology classes. Each of the nutrients in milk could be discussed in detail; The characteristics of water molecules could be compared to casein’s polar nature; The necessity of acidifying milk to make cheese is an excellent tie-in to the pH scale; Chymosin, an enzyme that aids in protein digestion, is another connection that can be drawn to the “lock-and-key” model. Cheesemaking organisms such as Lactococcus lactis, Streptococcus thermophilus, and Penicillium roqueforti offer real-life examples of beneficial bacteria that could be discussed in a Characteristics or Study of Life Unit. That’s the same unit where students often also learn about the cell membrane, the bi-lipid layer of cells. Here, casein micelles have points of commonality since they are hydrophilic in milk and become hydrophobic during the making of cheese. When Cheese Melts Cheese is also an emulsion of dairy fat and water. It is held together by a network of proteins. In cooler temperatures, that dairy fat remains a solid. At about 90°F the fat reaches a liquid state and the cheese becomes more pliable. Some cheeses begin to bead with "sweat" if they're left out at room temperature. Raise the temperature another 40-90 degrees and the bonds that joined the caseins together start to break and an oil slick forms.  Depending on the ratio of water to fat, as well as the strength of the protein network, some cheeses are “meltier” than others. Younger, high-moisture cheeses like mozzarella and brie melt better than drier grating cheeses like Parmesan or Pecorino-Romano. But even cheeses that melt well will cook off their water content with excessive heat. Instead of a smoothly melted cheese, too much heat can produce a tough ball of casein proteins floating in grease. That means that the protein structure shrunk so much that it could not contain the fat. In the past you might have noticed this when you found a greasy slick on top of an overcooked pizza or a lasagna. In your science class, this example is the opportune time to talk about the denaturing of proteins and what causes that to happen! Sodium Citrate – Food AND Tissue Preservative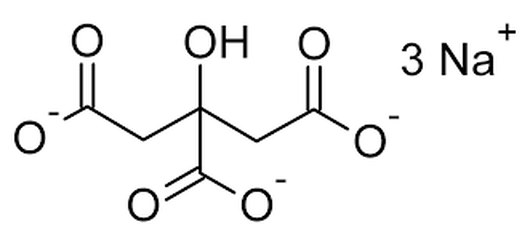 It was that greasy slick on top of over-heated cheese that food chemists Walter Gerber and Fritz Stettler wanted to avoid in 1911. A longer shelf-life was needed for shipping cheese during the bourgeoning global trade market in the early 1900s. When the researchers added a salt called sodium citrate to their cheese vat, the fat molecules stayed trapped among the proteins, even as the cheese melted and then cooled into a dense, easily sliceable form.  With the addition of sodium citrate, sodium ions replace calcium ions which help hold casein molecules together. This makes the caseins more negative, less hydrophobic, and more soluble. A looser, springier product is formed that is halfway between cheese and milk.  Sodium citrates are commonly used in food production. They regulate acidity in food and drinks, as well as emulsify oils to help them mix with other liquids. Interestingly, sodium citrate is also used to prevent donated blood from clotting in storage! This can be another great tie-in for an activity about blood transfusions! Kraft Introduced Velveeta in 1928 The Monroe Cheese Company in New York tasked Emil Frey with finding a use for its unsaleable broken cheese wheels in 1918. Frey’s creation, made with broken cheeses and other byproducts, had such a velvety-smooth consistency, he named it Velveeta. An independent business, the Velveeta Cheese Company, was incorporated in 1923 and was later sold to Kraft Foods. Kraft introduced Velveeta, with its own processed cheese formulation, to market on June 2, 1928.  The ingredient list in Velveeta has changed during the nearly 100 years it has been on the market. Cheese is not currently on the list. It is now sold in the US as a “pasteurized prepared cheese product” since the FDA does not maintain a standard of identity for that. And, certainly, the foodstuff is of dubious nutritional value. But it does make an interesting study for your science classroom!
0 Comments
Leave a Reply. |
AuthorGertrude Katz has spent over 30 years teaching K-12 public school students all major subjects. She has taught biology and education at the college level. The majority of her career has been spent instructing biology at the secondary level. Categories
All
|

 RSS Feed
RSS Feed